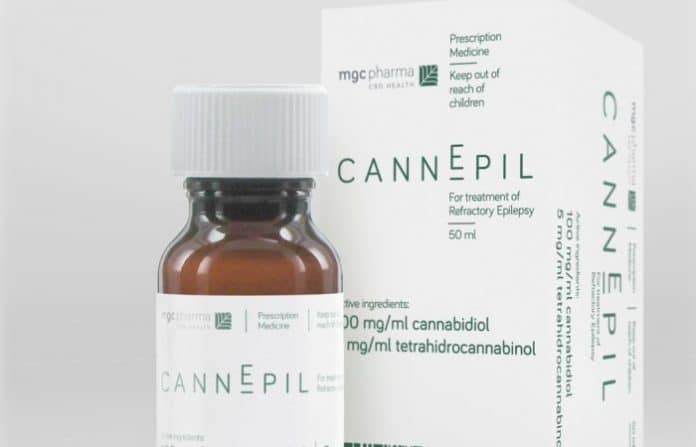
MGC Pharmaceuticals Ltd (ASX: MXC) has been authorised to make its CannEpil product available for supply in Australia under the Authorised Prescriber Scheme.
CannEpil is a primarily cannabidiol (CBD) based medication for the treatment of people with refractory epilepsy. These types of epilepsy are also referred to as uncontrolled, intractable, or drug-resistant. This label is usually applied when a patient doesn’t respond to medications after trialling two or three different types.
MGC Pharma says with the authorisation granted, it will begin commercial-scale production of CannEpil at its EU GMP certified facility in Slovenia immediately.
“This is a significant achievement for us and we are very excited at the prospect of ramping up production to a commercial scale and bringing CannEpil to Australia,” said Roby Zomer, the company’s Co-founder and Managing Director. “This important step validates MXC’s seed-to-pharma strategy with the start of commercial revenues from our first Investigational Medicinal Product offering.”
The news was well-received by Epilepsy Action Australia.
“This is a significant milestone for epilepsy patients and other patient communities. It is another important step in improving access to potentially life-changing medications, and part of a global trend to recognize the valid medical benefits of cannabis,” said the organisation’s CEO, Carol Ireland
It looks as though the company will make good on an announcement made in November last year that CannEpil will be available here in 2018. MGC says it is expected to be available for supply in Australia by December.
Zelda Consolidating Efforts
In other cannabusiness news from Australia yesterday, Zelda Therapeutics Limited (ASX:ZLD) says it has completed a strategic review of its operations and decided to halt clinical operations in Chile so that it can focus on clinical trials in the USA and Australia.
“Companies evaluating license deals usually put greater weight on US and Australian-based clinical trials so it makes sense to focus on these markets,” said Managing Director Dr Richard Hopkins.
Among its Australian activities is a trial involving the use of medical cannabis in treating insomnia. In the USA, it has partnered with Children’s Hospital of Philadelphia (CHOP) to study cannabinoid pharmacology, with a focus on the potential for treating autism.