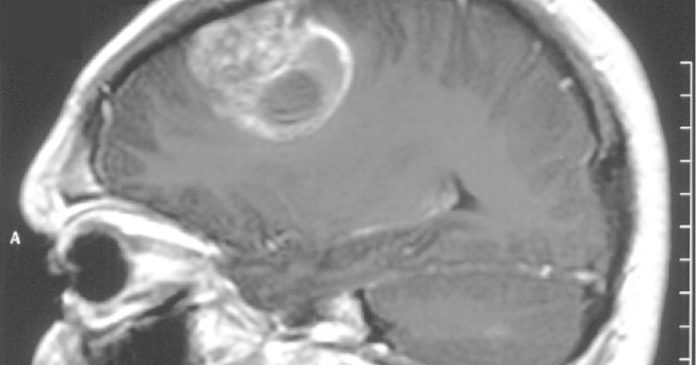
A cannabis-based medication already in use for another condition will be trialed to help treat the most aggressive form of brain tumour – recurrent glioblastoma.
In the UK, a major trial of Sativex is to kick off to assess whether adding it to chemotherapy treatment could extend life of patients with recurrent glioblastoma; which has an average survival of less than 10 months.
Sativex is an oromucosal (mouth) spray containing nabiximols; a cannabis extract primarily containing THC and CBD as the active ingredients along with lesser amounts of other cannabinoids. It’s one of only two cannabis medications currently approved for use in Australia by the Therapeutic Goods Administration, and only for treatment of moderate to severe spasticity associated with multiple sclerosis at this point.
“Glioblastoma brain tumours have been shown to have receptors to cannabinoids on their cell surfaces, and laboratory studies on glioblastoma cells have shown these drugs may slow tumour growth and work particularly well when used with temozolomide,” said Professor Susan Short, principal investigator on the new trial.
Researchers at Colorado State University last year suggested cannabidiol could prove useful in tackling glioblastoma as a result of their laboratory research.
The trial will occur in 15 NHS hospitals, following promising results from an earlier phase I study involving 27 patients. That study found more Sativex patients alive after one year compared to those in the placebo group.
More than 230 patients will be recruited across the UK for the trial, which is hoped to start next year. Two thirds of the participants will be given temozolomide and Sativex, while one third will be given temozolomide plus placebo.
The phase II trial, which will run over three years, is being funded by The Brain Tumour Charity and co-ordinated by the Cancer Research UK Clinical Trials Unit at the University of Birmingham. However, the trial will be led by the University of Leeds.
But there is a major challenge. The Brain Tumour Charity has seen its income drop by more than a quarter in the past year due to the COVID situation and has launched an appeal to raise the £450,000 needed to open the trial as soon as possible.