GW Pharmaceuticals estimates its net product sales for 2020 to have reached approximately USD $526 million – and its cannabidiol medicine made up the lion’s share.
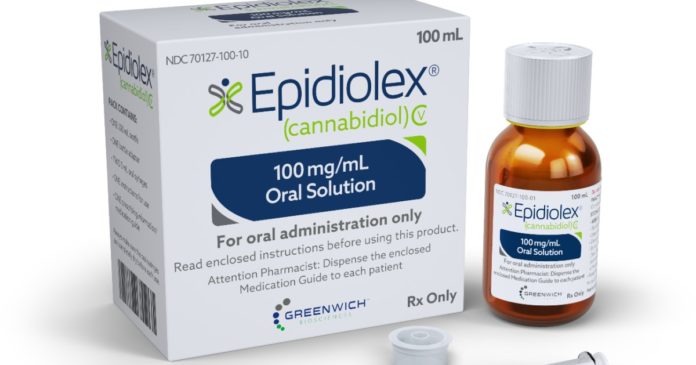
GW Pharmaceuticals produces a CBD product called Epidiolex, which is marketed under the name Epidyolex in the EU and Australia.
Epidiolex was the first CBD medicine approved by the USA’s Food and Drug Administration. It then went on to get approval in the European Union for the treatment of Lennox‑Gastaut syndrome and Dravet syndrome. In September last year, Australia’s Therapeutic Goods Administration (TGA) approved Epidyolex for the treatment of seizures associated with LGS or Dravet syndrome in patients two years of age and older.
In its preliminary, unaudited net product sales figures for 2020, GW Pharma notes Epidiolex raked in approximately $510 million for the full year. Just as a comparison, it was around this time last year the company reported approximately $309 million in net product sales for the year ended December 31, 2019, with Epidiolex accounting for $296 million.
“Epidiolex sales increased by over 70% in 2020 despite the challenges of COVID-19, reflecting the positive impact this medicine has on patients as well as the performance of our commercial team,” said GW’s Chief Executive Officer Justin Gover. “We remain encouraged by our patients’ experience on this product, as demonstrated by high persistence and refill rates.”
That persistence comes at a high price – quite literally. Epidiolex costs around USD$32,500 annually and whether it’s covered by medical insurance or not, at the end of it all someone has to pay for it; whether it’s taxpayers or fund members.
Looking at 2021, Mr. Gover says the company’s goals include driving growth in Epidiolex and advancing a number of trials for nabiximols in the treatment of MS spasticity. Nabiximols is a specific cannabis extract containing THC and CBD, and was the first natural cannabis plant derivative to gain market approval in any country.
Mr. Gover also mentioned he expects the company’s first product candidate based on synthesized cannabinoid molecules to enter Phase 1 trials this year. Among various other activities in 2021, GW will conduct placebo-controlled trials of both CBDV (cannabidivarin) and CBD for managing autism.