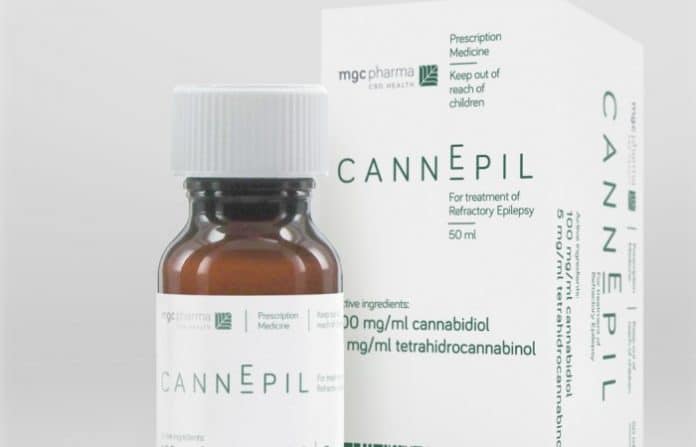
Australia’s MGC Pharmaceuticals has announced a definitive supply agreement that will see its first epilepsy medicine in the hands of Australian patients.
The company has inked a deal with Australian pharmaceutical distributor HL Pharma to bring MGC’s cannabidiol and tetrahydrocannabinol based CannEpil to the land down under.
While MGC is an ASX listed company, CannEpil will be manufactured at its Slovenian facility, which is currently undergoing GMP certification.
MGC says CannEpil is an affordable epilepsy product; but perhaps that should have read “more affordable”. The company states:
“Revenues from only the initial registered patients is expected to be approximately $1m in year 1, which is currently is less than 100 but expected to grow significantly in 2018/19.”
Even at 100 patients, if MGC is expecting $1 million in the first year, that translates to a cost of $10,000 a patient. It’s hardly cheap, but it is more affordable than some other medications for drug-resistant epilepsy.
“We now have a clear view on the pathway to the commencement of an Australian sales pipeline and revenue stream for our medical cannabis products which is a key milestone for our Company and is further evidence of the strong momentum that we are building through the execution of revenue generating agreements,” said Roby Zomer, MGC’s co-founder and CEO.
The company says least five doctors in Australia have already formally registered interest in prescribing CannEpil and MGC is also working closely with Epilepsy Action Australia (EAA).
Drug-resistant epilepsy (DRE), also known as refractory epilepsy or pharmacoresistant epilepsy, accounts for approximately 30% of the estimated 25,000 cases of epilepsy diagnosed in Australia each year.
As well as medicinal applications, MGC is also involved in cannabis-based cosmetics through its subsidiary, MGC Derma, and has been making solid headway on that front as well.
Last month, MGC Pharmaceuticals announced the signing of a $40 million agreement that will see white-label versions of its cannabidiol cosmetics products supplied to a Korean cosmetics manufacturer. On November 14, the company confirmed receipt of its first formal purchase order from Varm Cosmo.
MGC Derma is a joint venture between MGC Pharmaceuticals and Dr. M. Burstein Ltd. MGC Pharmaceuticals owns 51% and holds management control.