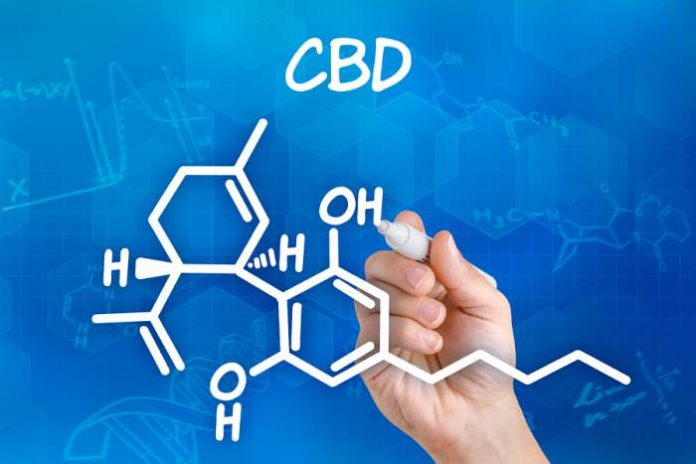
Results from another study indicate cannabidiol (CBD) can contribute to a significant reduction in the frequency of seizures in Lennox-Gastaut patients.
Lennox-Gastaut Syndrome (LGS) is a severe form of epilepsy that involves multiple and different types of seizures. It accounts for 2 to 5% of childhood epilepsies and is very difficult to manage with conventional seizure medications.
Last year, we covered the results of a study involving a medication based on the cannabinoid CBD indicating it was useful in treating the disorder.
Results just released from another trial, conducted by pediatric neurologist Dr. Saul Garza Morales, have been just as encouraging.
From January of 2016 to February this year, 45 patients diagnosed with Lennox-Gastaut Syndrome received progressive doses of up to 5-7 mg of cannabidiol per kg of body weight for at least 6 months after the study began. Of the original 45 participants, 39 patients saw the trial through to the end.
Of this group, there was a 50 percent or greater reduction in seizures in 84 percent of the cases and 53 percent of the cases saw an overall reduction in seizure activity of more than 75 percent. In 17 percent of the patients, there was a complete cessation of seizures for at least 4 months.
Other benefits were also observed, including increased alertness in 43 percent of patients, improved social interaction in 40 percent, and increased attention in 30 percent.
None of the participants suffered any serious side effects – this is very important distinction from conventional treatments.
The trial involved the use of the rather oddly named “Real Scientific Hemp Oil-X”, which contains no tetrahydrocannabinol (THC).
“This is such encouraging news that children with such a severe form of epilepsy now have an option for relief as opposed to a prior bleak diagnosis that offered no signs of beneficial treatment, once the epilepsy moves to the refractory (drug resistant) phase,” said Dr. Stuart Titus, CEO of Medical Marijuana, Inc.; parent company of the company that manufactures the oil.
“Dr Garza’s study is a huge victory for botanical CBD as opposed to the pharmaceutically developed alternative.” Synthetic CBD is being used in an epilepsy trial in New Zealand.
More from the study can be viewed here (PDF).
The evidence is mounting that cannabidiol, while not a panacea, could improve the lives of millions of people around the world suffering from various chronic and serious conditions.